Abstract
Background Central nervous system (CNS) relapse is a serious complication of diffuse large B-cell lymphoma (DLBCL). Although prophylactic high-dose methotrexate (HD-MTX) is used to reduce the risk of CNS relapse, recent studies have reported the ineffectiveness of HD-MTX in preventing CNS relapse in DLBCL patients. However, these studies might have been influenced by a potential bias in patient selection for MTX prophylaxis.
Aim We aimed to evaluate the prophylactic effect of HD-MTX on CNS relapse by direct comparison with intrathecal methotrexate (IT-MTX) in DLBCL patients at high risk for CNS relapse. The patients were screened using defined patient selection criteria and treated with a uniform CNS prophylaxis.
Method This multicenter retrospective study was conducted at six institutions. Patients diagnosed with DLBCL at a high risk of CNS relapse between January 2003 and December 2020 were treated with frontline R-CHOP, R-CHOP-like, or dose-adjusted EPOCH-R chemotherapy. All DLBCL patients were initially screened using defined patient selection criteria for the risk of CNS relapse: (1) involvement of the testis, breast, adrenal gland, kidney, bone, or paranasal sinus, and (2) CD5-positive DLBCL identified by immunohistochemistry. Patients at high risk for CNS relapse received CNS prophylaxis after achieving an overall response to initial chemotherapy with IT-MTX between 2003 and 2013 and HD-MTX between 2014 and 2020. IT-MTX (15 mg/body) with hydrocortisone (25 mg/body) was administered four times, once weekly. HD-MTX (3 g/m2/cycle) was administered for two cycles at two-week intervals. The HD-MTX dose in elderly patients was modified to two cycles of HD-MTX (1.5 g/m²) for patients aged 61-70 years, and no HD-MTX was administered to patients older than 70 years between 2003 and 2018. Since 2019, all eligible patients younger than 80 years have received two cycles of HD-MTX (3 g/m²), and the dose has been individually modified for patients over 80 years of age. CNS relapse was defined as an isolated relapse in the CNS, excluding cases of CNS relapse occurring simultaneously with or after systemic relapse.
Result Among 132 eligible patients (98 given HD-MTX and 34 given IT-MTX), the median age was 62.5 years (range 22-84), 64.4% were male, 17.4% had ECOG-PS ≥2, 52.3% had advanced clinical stage (III/IV), 47.0% had elevated serum LDH, and 34.1% had two or more extranodal site involvements, with the most common site being the testis (32.6%). Regarding the risk factors, 23.5% had a high CNS-IPI score, 11.4% had a high NCCN-IPI score, and 31.1% were positive for CD5. In terms of frontline chemotherapy regimens, 83.3% received R-CHOP or R-CHOP-like regimens, and 16.7% received EPOCH-R.
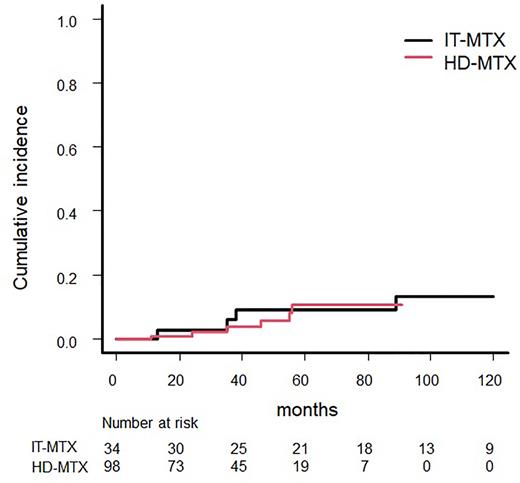
The median follow-up for the entire cohort was 52 months (range 9-174 months), and 11 patients had isolated CNS relapse: 6 (6.1%) in the HD-MTX group and 5 (14.7%) in the IT-MTX group. All CNS relapses involved brain parenchymal lesions. The median time until CNS relapse was 38 months (range 11-122; HD-MTX 11-56, IT-MTX 13-122 months). The incidence of CNS relapse at 3 years was 3.9% (95% CI 1.0-10.1) in the HD-MTX group and 6.1% (95% CI 1.0-17.9) in the IT-MTX group (P = 0.93). In the overall population, the 3-year PFS was 86.3% (95% CI 77.0-92.1) in the HD-MTX group and 90.8% (95% CI 73.9-96.9) in the IT-MTX group (P = 0.75). The 3-year OS was 92.8% (95% CI 84.5-96.7) in the HD-MTX group and 91.2% (95% CI 75.1-97.1) in the IT-MTX group (P = 0.67).
A total of 81 patients were matched in a propensity score-matched analysis. CNS relapse occurred in eight patients (9.9%). There was no significant difference in the risk of CNS relapse after 3 years between the prophylaxis routes (4.6% HD-MTX vs. 7.6% IT-MTX, P = 0.86).
Prophylaxis-related toxicity was reported in 45 (34.1%) patients with a frequency of toxicity in the HD-MTX and the IT-MTX groups of 39.8% and 17.6%, respectively (P = 0.02). There were 8 events in the HD-MTX group with a grade ≥3, and 2 events in the IT-MTX group with a grade ≥3.
Conclusion Even in our patient population selected by our defined selection criteria who had received uniform CNS prophylaxis, we found no difference in CNS relapse between the HD-MTX and IT-MTX populations. The results were similar after adjusting for background factors with a propensity score-matched analysis. However, because CNS relapse is a rare event, a larger number of patients and longer follow-up periods are needed to confirm this conclusion.
Disclosures
Akimoto:Chugai/Roche: Honoraria. Miyazaki:Chugai/Roche: Honoraria; AbbVie: Honoraria; Bristol Myers Squibb: Honoraria; Takeda: Honoraria; Symbio: Honoraria; Janssen: Honoraria; Asahi Kasei: Honoraria; Novartis: Honoraria. Takahashi:Takeda: Speakers Bureau; Ono: Speakers Bureau; Janssen: Speakers Bureau; Chugai/Roche: Speakers Bureau; Kyowa Kirin: Speakers Bureau; Nippon Shinyaku: Speakers Bureau; Eisai: Speakers Bureau; Mundi pharma: Speakers Bureau; Symbio: Speakers Bureau; Astra zeneca: Speakers Bureau; Meiji seika pharma: Speakers Bureau. Fujimaki:CSL Behring K.K.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Meiji Seika Pharma Co., Ltd.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Nippon Shinyaku: Honoraria; Janssen Pharmaceutical KK: Honoraria; AbbVie GK: Honoraria; Otsuka Pharmaceuticals: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Bristol-Myers Squibb: Honoraria. Sakai:Chugai Pharmaceutical Co.: Research Funding; Kyowa Hakko Kirin Co.: Honoraria, Research Funding; Taiho Pharmaceutical Co: Research Funding; Astra Zeneca plc.: Honoraria; Takeda Pharmaceutical Co.: Honoraria. Fujisawa:Chugai/Roche: Research Funding; Shionogi: Research Funding; Kyowa Hakko Kirin: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Asahi-Kasei Pharma: Research Funding; Astellas: Honoraria, Research Funding; Pfizer Japan Inc.: Honoraria, Research Funding; Bristol-Myers-Squibb: Honoraria; Nippon Shinyaku: Honoraria; Novartis KK: Honoraria; MSD K.K.: Honoraria; Sanofi: Honoraria; Janssen: Honoraria; SymBio Pharma: Honoraria; AstraZeneca: Honoraria; CSL Behring K.K: Honoraria; Meiji Seika Pharma: Honoraria; AbbVie Inc: Honoraria. Nakajima:Novartis: Speakers Bureau; Daiichi-Sankyo, Inc: Research Funding, Speakers Bureau; Nippon Shinyaku: Research Funding; Celgene Corporation: Research Funding; Astellas Pharma: Other: Scholarship donation; Chugai/Roche: Other: Scholarship donation, Research Funding; Asahi Kasei Pharma Corporation: Other: Scholarship donation; Takeda: Other: Scholarship donation; Eisai: Other: Scholarship donation; Pfizer: Other: Scholarship donation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal